 |
|
 |
Two-photon Ca2+ imaging of GCaMP6s neurons in A1
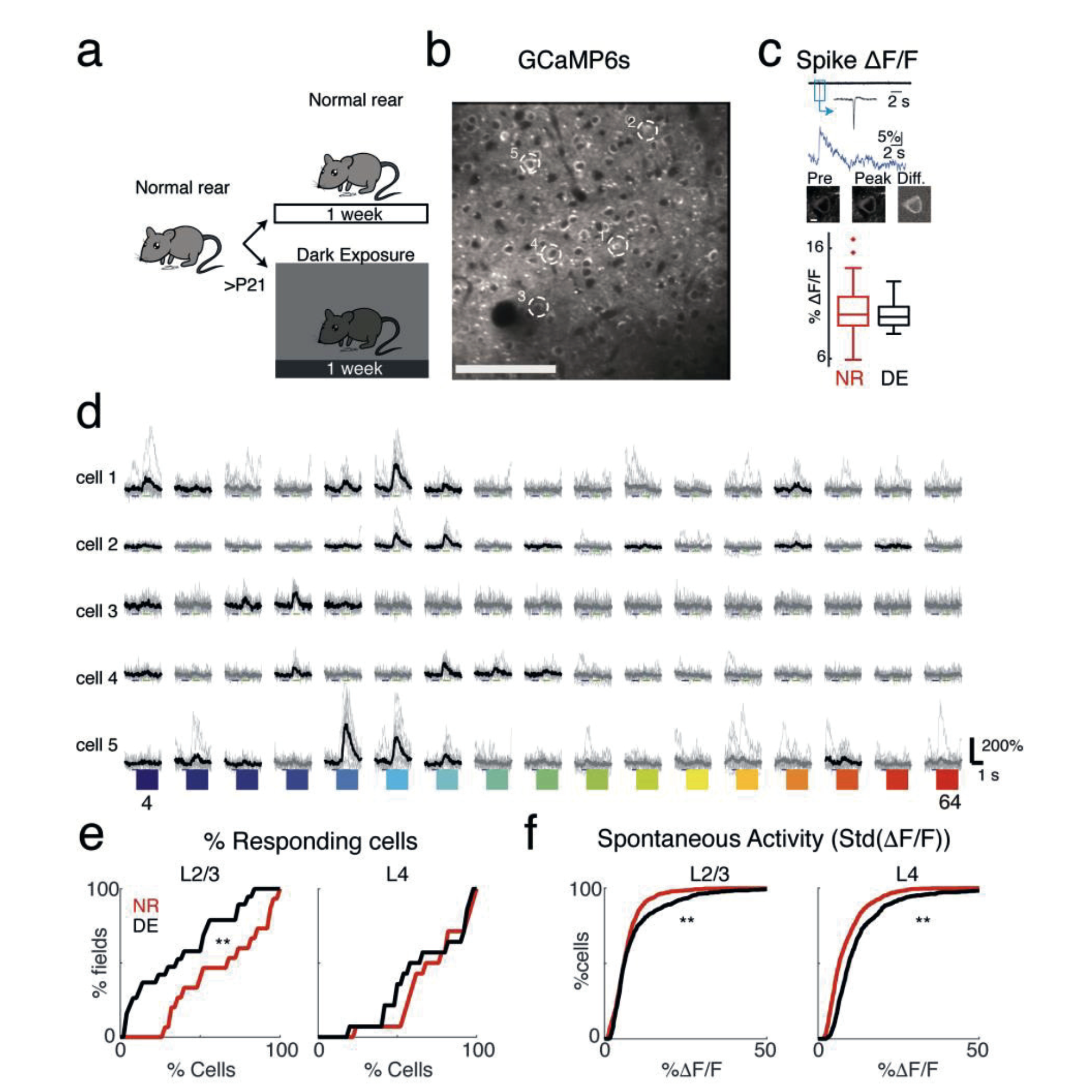
(a) Experimental paradigm. Animals are raised in normal environments until at least P21. Animals then either stay in the normal lighted environment or are dark exposed for 7 days. (Cartoon by Zara Kanold-Tso.) (b) Imaged field with GCaMP6 expressing neurons. Exemplar neurons indicated by white circles. Scale bar 100 micrometers. (c) Cell-attached patchrecordings in vitro from GCaMP6S-expressing neurons. Top row shows current trace –with inset showing magnified action potential. Middle row shows the corresponding Ca2+rise (ΔF/F) in response to one action potential. Bottom row shows the corresponding two 535 photon fluorescence images: first image is of cell preceding spike, middle image is of cell1 9 at peak of fluorescent response, and third image shows the difference (scale bar = 5 μm). Boxplots shows median and interquartile range of fluorescent-evoked responses to one spike in control and DE mice. DE does not alter the amplitude of spike-induced fluorescence transients (mean ΔF/F ± SEM per spike: NR= 10.25% ± 0.29%, n=62 spikes;DE= 9.97% ± 0.20%, n=37 spikes; Two-sample Kolmogorov-Smirnov test, p= 0.16). (d)Sound-evoked fluorescence traces in 5 exemplar cells (indicated in b). Black lines indicate mean trace for responses that passed the significance criterion (ANOVA p < 0.001), whilethin gray traces show individual trials. Colors indicate tone frequency 4-64kHz. (e) Fraction of responsive cells decreases in L2/3 following dark-exposure (mean ± standard deviation,NR= 64.2% ± 26.9, DE= 37.4% ± 28.4, Wilcoxon rank-sum test, p= 0.0167) with no change in L4 (NR= 72.1% ± 22.0, DE= 66.9% ± 25.4, p= 0.35). (f) Spontaneous activity, as measured by standard deviation of the baseline in ΔF/F traces, increased in L4 and L2/3after DE (NR median ± iqr L2/3: 6.1 ± 4.7, DE L2/3: 6.2 ± 6.2, p = 0.0018; NR L4: 6.6 ± 6.7,DE L4: 9.6 ± 7.9; Wilcoxon rank-sum test, p < 10-28;). Figure courtesy Kanold Group |
|
Brain plasticity research by ISR-affiliated Professor Patrick Kanold’s (Biology) group shows that even a brief period of visual deprivation in mice can alter tone-evoked responses of neurons as well as frequency representation in multiple layers of the primary auditory cortex. This new research offers evidence that cross-modal sensory experience has the power to alter the brain’s network circuitry and population dynamics even into adulthood.
“Temporary visual deprivation causes decorrelation of spatio-temporal population responses in adult mouse auditory cortex” was published in the journal eNeuro in November. The paper’s authors include Kanold’s former advisee Krystyna Solarana (Ph.D. Biology 2016), a communications specialist with USAID in Tajikistan; two of his current Ph.D. students, Ji Liu and Zac Bowen; and Hey-Kyoung Lee, a professor of neuroscience at Johns Hopkins University.
About the research
Within the brain, sensory cortices can rewire in response to environmental input, especially during critical periods in development. When a sensory modality, such as sight, is lost, the brain’s plasticity allows the remaining senses to compensate. Research has shown that humans experiencing vision loss from birth exhibit cross-modal perceptual enhancement of hearing, including improved sound localization abilities, frequency discrimination performance, and auditory spatial tuning.
Although within-modality sensory plasticity is limited to early developmental periods, cross-modal plasticity can occur even in adults. Compensatory plasticity in the auditory domain is not only limited to early onset or prolonged vision loss, but can be observed when vision loss occurs later in life and over a shorter period. In humans, late-onset blindness can enhance auditory localization, and even brief periods of visual deprivation can transiently improve auditory perception by enhancing sound source segregation. However, the circuit basis for these perceptual enhancements is unclear.
Previous studies have shown that transient visual deprivation (dark exposure) in adult mice improves the frequency selectivity and discrimination of neurons in their brains’ primary auditory cortex. The Kanold group investigated whether dark exposure alters network activity, and found that one week of dark exposure in adult mice increased the sound evoked responses and frequency selectivity of related neurons, and sharpened tuning curves.
The paper shows that a brief period of visual deprivation in mice can alter the tone evoked responses of neurons as well as the frequency representation in multiple layers of their brains’ primary auditory cortex—showing the tuning of auditory cortex neurons can be altered even after the brain’s critical development period. The researchers also showed that pairwise correlations are decreased, indicating a sparsification of the evoked responses in the auditory cortex. These results add to mounting evidence that cross-modal sensory experience has the power to alter the brain’s network circuitry and population dynamics even into adulthood.
For a more complete news story about this research, read this account from UMD's College of Computer, Mathematical and Natural Science.
Related Articles:
New UMD Division of Research video highlights work of Simon, Anderson
How does the brain turn heard sounds into comprehensible language?
Internal predictive model characterizes brain's neural activity during listening and imagining music
Shamma, colleagues publish in Journal of Neuroscience
UMD auditory cortex research featured in Nature Neuroscience
Stop—hey, what’s that sound?
Waks, Shapiro receive NSF EAGER grant to test spintronic devices
‘Priming’ helps the brain understand language even with poor-quality speech signals
Two ECE Graduate Students Win 2023 UMD Three Minute Thesis Competition
Autism Research Resonates in Hearing-Focused Project
November 26, 2019
|

