 |
|
 |
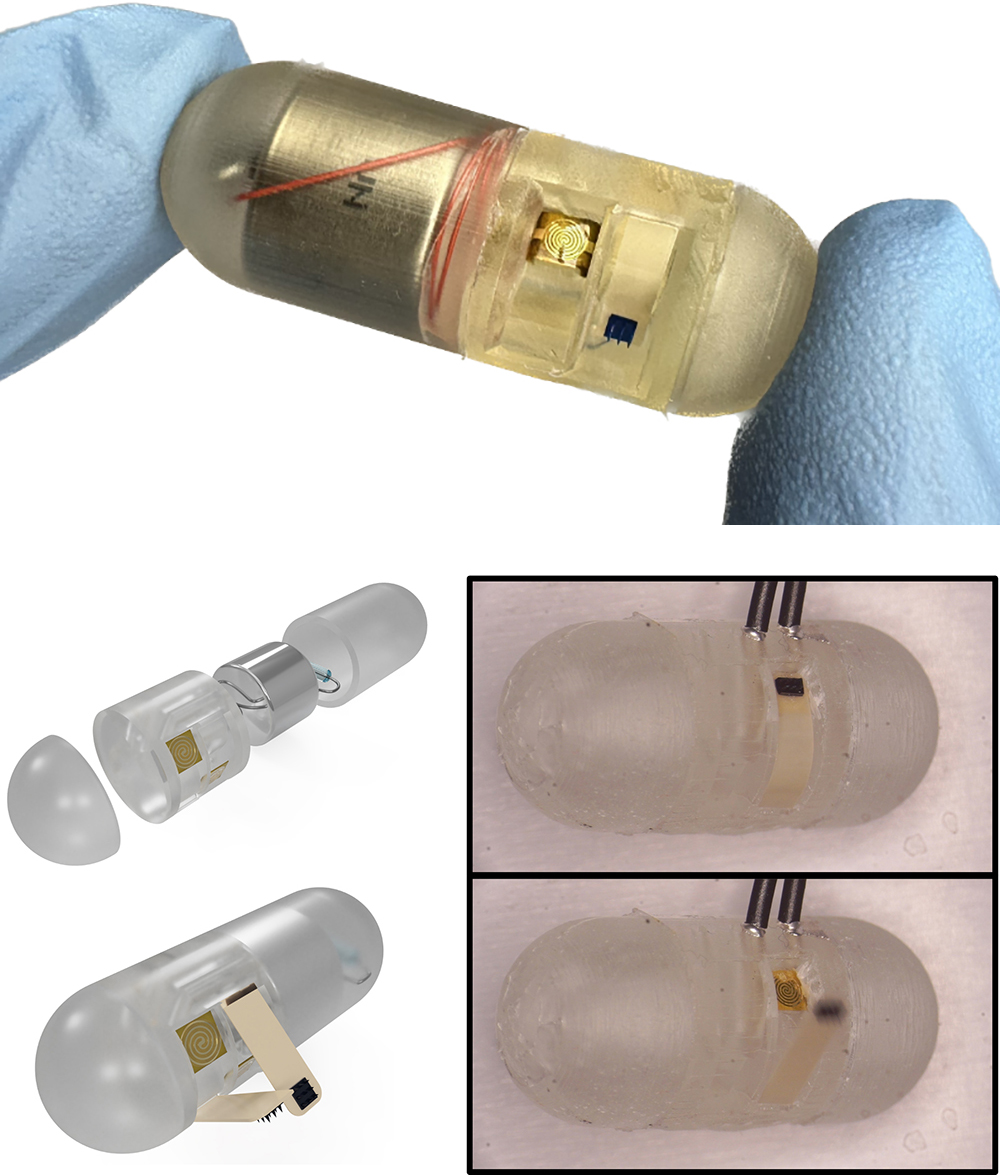
Above, the current capsule. Below, left top: An exploded diagram view of the capsule components. Below, left, bottom: After heat activation, the cantilevers open and the microneedles are ready to deploy. Below, right: The capsule shown during testing. |
|
The newest version of an ingestible capsule being developed by the University of Maryland (UMD) MEMS Sensors and Actuators Laboratory (MSAL) appears in the Aug. 16, 2024 issue of Device, a Cell Press journal. “Magnetically triggered ingestible capsule for localized microneedle drug delivery” describes a remotely triggerable local drug injection system for the gastrointestinal (GI) tract. Although not yet available to the medical community, this advance points to the day clinicians could use an ingestible capsule to better treat intestinal diseases. The capsule can be directly controlled with external magnets, using dissolvable microneedles that provide adaptable, efficient, fast, localized drug delivery.
Materials Science and Engineering Ph.D. student Joshua Levy is the first author. In early June 2024, he won the Janusz Bryzek Abundance through MEMS Award at the Workshop on Solid-State Sensors, Actuators, and Microsystems (aka the Hilton Head Workshop) for related work. Other authors include Fischell Department of Bioengineering graduate student Michael Straker, 2024 Electrical and Computer Engineering Ph.D. recipient Justin Stine, UMD Research Associate Luke Beardslee (ISR) and Herbert Rabin Distinguished Chair in Engineering, Professor Reza Ghodssi (ECE/ISR). Levy, Straker, Stine and Ghodssi are also affiliated with the Robert E. Fischell Institute for Biomedical Devices. Dr. Ghodssi is the advisor to Levy, Straker and Stine.
The device and improved disease treatment
In their article, the authors demonstrate a scalable, ingestible capsule device that features flexible polyetherketone (PEEK) cantilevers, under which are microneedles that can deliver a drug payload to specific GI tract locations. When the capsule reaches its target, its resistive heating element is activated to melt a binding adhesive. This triggers the deployment of cantilever actuators that insert the drug-laden microneedles into the intestinal tissue. The microneedles dissolve after use.
The device can be remotely triggered with a handheld magnet in 2.91 ± 0.48 seconds.
“This technology is a major step forward for our research because it allows precise control over the timing and location of drug delivery from outside the body,” Joshua Levy said.
The ingestible capsule system holds significant potential for future medical practice. Delivering drugs in a targeted, local way could substantially increase the efficiency and effectiveness of GI therapies. Currently used oral drugs spread systemically throughout the body and produce widespread side effects. Capsule-delivered treatments could be more effective and comfortable for inflammatory bowel disease (IBD) and Crohn’s disease patients, encouraging greater compliance with drug regimens.
Future research
Currently, the capsule is 13 mm in diameter, but all its components—cantilevers, batteries, microneedles, heaters and packaging—can continue to be further miniaturized. The technology has the potential to operate as a standalone device, but auxiliary devices like PillCams could be used in conjunction with the capsule to identify potential intestinal treatment sites, and fluoroscopes and ultrasounds could help pinpoint the capsule’s location in vivo.
“Ingestible capsules are promising devices for demonstrating miniature sensors and actuators' novel characteristics and impacts through advanced manufacturing processes and systems integration techniques,” Reza Ghodssi said. “This work is an exciting example of how MEMS sensors and actuators technology could significantly impact future biomedical devices for drug delivery applications.”
Development journey
In development since 2017, the ingestible capsule has been supported by a National Science Foundation (NSF) EAGER grant, the NSF ECCS Program, UMD TerrapinWorks, the Nanocenter FabLab, and the A. James Clark School of Engineering’s Mid-Career Doctoral Fellows Program. Innovations in the device’s development include:
Related Articles:
Dropping an anchor for better GI tract disease treatment
Research Paper and Cover Art Now Feature Article in Journal
Ingestible Capsule Advances May Lead to Earlier Detection of Diseases
Ingestible Capsule Technology Research on Front Cover of Journal
Gut Health Monitoring Gas Sensors Added to Ingestible Capsule Technology
New ‘FRRB’ packaging technology may solve an ingestible capsule challenge
Fischell Fellowship advances visiting assistant professor’s work
Adjustable Drug Release Marks New Milestone in Ingestible Capsule Research
Ghodssi invited speaker at NIMH workshop on sensor technologies to capture the complexity of behavior
MSAL’s work on serotonin characterization and detection results in two journal covers
July 2, 2024
|

