|
 |
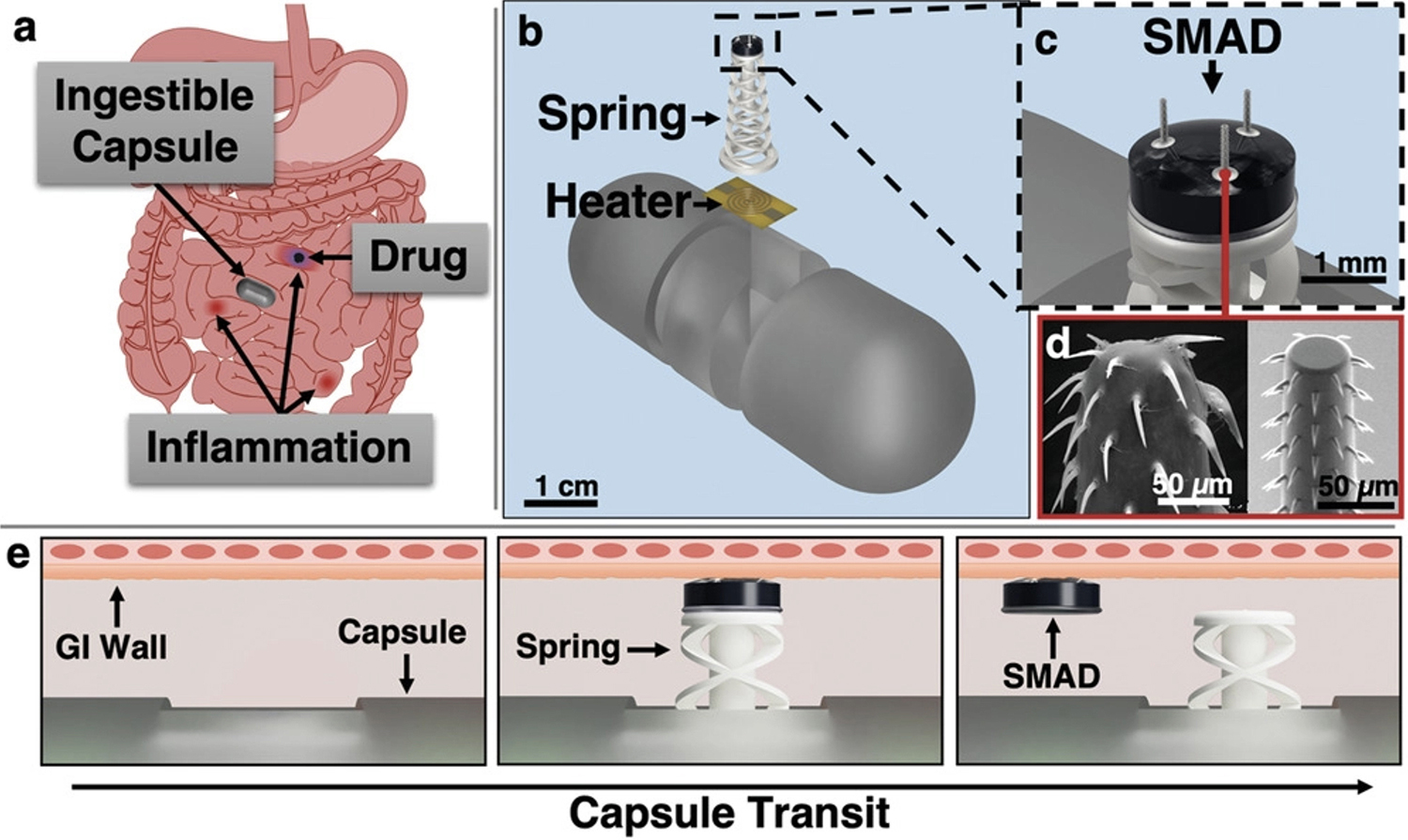
Figure 1 from the paper. (Click for larger view) This figure details the capsule's actuation and SMAD delivery principle. a) Capsule transits through a GI tract with inflammatory lesions and delivers the SMAD to an inflammatory site for prolonged release of a topical therapeutic agent (blue). b) Actuator deployment utilizes a resistive heating element to fire a spring actuator and impart the drug-loaded SMAD into the GI tissue. c) CAD rendering of the SMAD on the actuator. d) Spiny microneedles are designed to mimic a spiny-headed worm proboscis for enhanced anchoring in tissue: Reproduced under terms of the CC-BY license. e) Upon sensed or external command for SMAD delivery, current passes through the heating element, melting the polycaprolactone binder, and firing the spring. The SMAD is then imparted into the tissue and removes as the capsule translates through the intestinal tract. |
|
Some 3.1 million people in the United States suffer from chronic gastrointestinal (GI) autoimmune disorders like inflammatory bowel disease, Crohn's disease and ulcerative colitis. Medical science has made substantial advances in treating these health grand challenges in the last few decades, largely through “systemic” therapies like pills, injections and infusions. Unfortunately, as systemic therapies diffuse throughout the body, their effectiveness also diffuses. Medicine can’t be targeted to the inflammatory lesions that characterize these gut diseases. In addition, these treatments produce substantial side effects.
Scientists have been working for years to develop new ways to deliver inflammatory disease medication. One idea seems to be straight out of science fiction: ingestible, minimally invasive capsules that can detect, monitor, and treat chronic problems from inside the GI tract.
There have been many recent advances in this promising technology. These capsules can perform GI imaging, gas sensing, lesion biopsy, and drug delivery. They can be commanded remotely through WiFi and a phone app. Still, a number of practical problems need to be solved—like how to keep the capsule in place to deliver medicine amidst the constant churning of the digestive system.
At the University of Maryland, Professor Reza Ghodssi’s (ECE/ISR) MEMS Sensors and Actuators Laboratory (MSAL) has published new research in the Dec. 4, 2022 issue of the journal Advanced Materials Technologies. “Thermomechanical Soft Actuator for Targeted Delivery of Anchoring Drug Deposits to the GI Tract” was written by Materials Science and Engineering Ph.D. student Joshua Levy, Bioengineering Ph.D. student Michael Straker, Electrical and Computer Engineering Ph.D. student Justin Stine, University of Maryland Research Associate Luke Beardslee, alum Vivian Borbash (ECE B.S. 2022), and Ghodssi. Ghodssi is the Ph.D. advisor for Levy, Straker and Stine; all are associated with the Robert E. Fischell Institute for Biomedical Devices.
This new research demonstrates a compact mesoscale spring actuator that can anchor the capsule, allowing it to deliver a drug deposit to locations in the GI tract mucosa. The new mechanism works with existing ingestible capsule-based sensing and communication technologies and enables treatment based on detected GI biomarkers and external commands. With the ability to stay in place for a sustained period of time, the capsule can deliver multiple doses of medication as needed.
MSAL’s history of ingestible capsule innovations
MSAL has been working on capsule development for five years. In January 2020 at the IEEE MEMS 2020 conference, an MSAL team presented work on biomimetic barbed microneedles. In March 2020 the group published a research review on ingestible sensors in the journal ACS Sensors. In April 2020 the lab’s proof-of-concept work on sensing in the duodenum was published in Lab on a Chip. In 2021, Joshua Levy won the Best Student Paper Award for developing a micro-needle platform for drug delivery. At the 2022 Transducers Research Foundation Hilton Head Workshop, the group presented work on integrated sensors for the capsules, as well as attachable drug delivery modules. Levy and Stine were nominated for Best Paper Awards. They gave oral presentations on the spring actuator/drug delivery research and on a proof-of-concept design for a capsule capable of electrochemical detection of hydrogen sulfide. In addition, the group filed a provisional patent in June 2022 for “Systems and Methods for Medical Device Anchoring” that covers anchoring for a broad array of medical devices.
On a related track, the MSAL team also has been developing an ingestible capsule that can monitor and model gut microbiome serotonin activity and help demystify the "gut-brain axis."
The new spring actuator
The present paper introduces the compact thermomechanical 3D-printable actuator and combines it with the first application of biomimetic barbed microneedles for drug delivery. This microneedle package is known as “SMAD,” for Spiny Microneedle Anchoring drug Deposit.
The newly designed 3D-printable spring actuator is a type of “wave spring.” Wave springs are used for components that need to travel a long distance when in use but take up little space beforehand—a necessity for ingestible capsule devices. This type of spring also provides better lateral stability than a standard conical coil spring.
On the capsule, the compressed spring is fixed in place with polycaprolactone, a biodegradable polyester with good resistance to water and oil and a low melting point of 60 °C. When it’s time to deploy the spring and propel its payload, the capsule’s tiny resistive heating element melts the polycaprolactone, releasing the compression and setting the spring in motion.
The drug-loaded SMAD, which uses the MSAL lab’s 2020 biomimetic barbed microneedle technology, is attached to the top of the spring actuator with polyethylene glycol (PEG), a water-soluble polymer that enforces the reliable release of the SMAD from the spring.
“Our innovation is an early example of using hybrid fabrication approaches that merge 3D printing with traditional microfabrication to create new and impactful devices,” first author Joshua Levy says. “We expect our work will help form the foundation of new forms of treatment, and that these devices eventually will lead to better therapies.”
In tests, the actuator deployed reliably and repeatedly. The system is compatible with sensing and communication technology currently being used in ingestible capsules. It provides closed-loop detection and enables prolonged dissolving therapeutic drug delivery to specific lesions in the GI tract for early, focused, and long-term treatment.
It is an improvement over current capsules that use passive mechanisms to support locational drug delivery via microneedles. Existing capsules do not permit fully closed-loop deployment in response to sensors or explicit commands, and only offer regional control over delivery.
“The GI tract is a passage through the body that influences who we are through its direct connections to the outside world,” says Professor Ghodssi. “This unique organ is susceptible to a number of health grand challenges, from cancer to IBD to neurodegenerative diseases, and mental health problems and metabolic diseases as well.”
“But currently medical experts have only a very limited set of mostly systemic therapies to identify, monitor and treat GI diseases. We hope that our emerging non-invasive capsule technology will be able to put another tool in the medical kit, one with fewer side effects and better targeted efficacy.
“Our work addresses only one of the promising research areas for this technology,” Ghodssi says. “We believe developing ingestible capsules is a frontier of research that requires an interdisciplinary team of doctors, engineers, biologists and data analysts to solve.”
Related Articles:
New features on ingestible capsule will deliver targeted drugs to better treat IBD, Crohn’s disease
University of Maryland Research is Redefining Health Care
Research Paper and Cover Art Now Feature Article in Journal
Ingestible Capsule Advances May Lead to Earlier Detection of Diseases
Ingestible Capsule Technology Research on Front Cover of Journal
Gut Health Monitoring Gas Sensors Added to Ingestible Capsule Technology
New ‘FRRB’ packaging technology may solve an ingestible capsule challenge
Fischell Fellowship advances visiting assistant professor’s work
Adjustable Drug Release Marks New Milestone in Ingestible Capsule Research
Ghodssi invited speaker at NIMH workshop on sensor technologies to capture the complexity of behavior
December 5, 2022
|

