|
 |
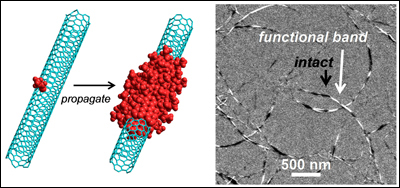
Left: The Billups-Birch alkylcarboxylation reaction allows functional groups to propagate down the CNT from points of pre-existing defects. Right: Electron microscopy shows ?banded? CNTs with distinct functionalized and intact regions along their lengths. Photo credits: Nature Communications. |
|
A team of University of Maryland nanotechnology researchers has solved one of the most vexing challenges hindering the use of carbon nanomaterials in increasing electrical energy storage efficiency in batteries or enhancing the fluorescence sensing capabilities of biosensors. The findings are published in the July 12 issue of Nature Communications.
The breakthrough research was led by Chemistry Assistant Professor YuHuang Wang and conducted in the Nanostructures for Electrical Energy Storage center (an Energy Frontier Research Center of the Department of Energy), Northwestern University, and the Maryland NanoCenter.
Carbon nanotubes (CNTs) have enormous potential. They are some of the most conductive structures ever made?highly efficient electrodes with enormous surface area. To take full advantage of these properties, however, CNTs must be soluble?that is, have the ability to be dispersed in a liquid environment or to evenly coat a solid composite material. Unfortunately, in their raw state CNTs are insoluble; they clump together rather than disperse.
For more than a decade, researchers have been developing new chemical processes to address this challenge. One idea has been to create permanent defects on the surfaces of CNTs and ?functionalize? them so they are soluble. Unfortunately, this also has the undesired side effect of quickly destroying the CNTs? electrical and optical properties.
Wang and his team have developed a new functionalization process for CNTs that delivers solubility and preserves electrical and optical properties. They purposefully functionalize defects on the tubes in useful?not random?places, creating strategic ?functional groups.? These carefully placed molecular groups allow CNTs to readily disperse while retaining their optical properties and ability to conduct electric current in large regions along the tube.
The challenge has been to control the chemical reactions that produce the functional groups on the CNTs. By using a chemical process called Billups-Birch reductive alkylcarboxylation, Wang?s team found they could progressively add new functional groups to the CNT wall in a controlled way without introducing unintended new defects.
When the CNTs are immersed in a chemical solution for a specific length of time, the functionalized groups on the nanotubes lengthen by a predictable amount. Each time the process is repeated, or as the time in the solution increases, the sections grow longer. When the CNTs are viewed under a special, high magnification electron microscope, it is evident that the functionalization has progressed lengthwise along the tube.
The propagation can initiate from either naturally occurring or intentionally introduced defects. Because the propagation mechanism confines the reaction and strategically controls where the functional groups grow, Wang?s team can produce clustered functional groups at a controlled, constant propagation rate. It is the first clearly established wet chemistry process that does so.
The breakthrough makes it possible to create new functional structures such as ?banded? nanotubes with alternating segments of functionalized and intact regions. The functionalized regions keep the CNTs from clumping, making them among the most water-soluble CNTs known. At the same time, the bands of intact, non-functionalized regions of the CNTs allow electrical and optical properties to be retained.
?This is important for the future use of these materials in batteries and solar cells where efficient charge collection and transport are sought,? Wang explains. ?These CNTs also could be used as highly sensitive biochemical sensors because of their sharp optical absorption and long-lived fluorescence in the near infrared regions where tissues are nearly optically transparent.?
?This is a major step towards building the controlled nanostructures needed to understand electrochemical science and its value for energy solutions,? says University of Maryland NanoCenter Director, Professor Gary Rubloff (MSE/ISR), a collaborator on the project.
The research team also includes theoretical chemist Professor George Schatz of Northwestern University, postdoctoral associates and graduate students Shunliu Deng, Yin Zhang, and Alexandra Brozena, who are equal contribution first authors, as well as Maricris Mayes, Parag Banerjee and Maryland NanoCenter staff member Wen-An Chiou.
Related Articles:
Company Co-Founded by Sergio Baron Delivers Ultra-Thin, Custom Shape Lithium Batteries
Gary Rubloff keynote speaker at IEEE nanotechnology conference
Alexander Pearse wins Dean's Doctoral Research Award
A nerve modeled on a battery
Gary Rubloff named Distinguished University Professor
Rubloff, Ghodssi to speak at TechConnect World Innovation Conference and Expo
Gary Rubloff to receive Senior Faculty Outstanding Research Award
Rubloff, Wu win UMD Invention of the Year Awards
Lithium-ion battery research profiled in DOE newsletter
Rubloff, Ghodssi featured in JVST-A special issue
July 11, 2011
|

